Abstract
Turnover of soil organic carbon (SOC) is strongly affected by a balance between mineral protection and microbial degradation. However, the mechanisms controlling the heterogeneous and preferential adsorption of different types of SOC remain elusive. In this work, the heterogeneous adsorption of humic substances (HSs) and microbial carbon (MC) on a clay mineral (nontronite NAu-2) during microbial-mediated Fe redox cycling was determined using time-of-flight secondary ion mass spectrometry (ToF-SIMS). The results revealed that HSs pre-adsorbed on NAu-2 would partially inhibit structural modification of NAu-2 by microbial Fe(III) reduction, thus retarding the subsequent adsorption of MC. In contrast, NAu-2 without precoated HSs adsorbed a significant amount of MC from microbial polysaccharides as a result of Fe(III) reduction. This was attributed to the deposition of a thin Al-rich layer on the clay surface, which provided active sites for MC adsorption. This study provides direct and detailed molecular evidence for the first time to explain the preferential adsorption of MC over HSs on the surface of clay minerals in iron redox processes, which could be critical for the preservation of MC in soil. The results also indicate that ToF-SIMS is a unique tool for understanding complex organic–mineral–microbe interactions.

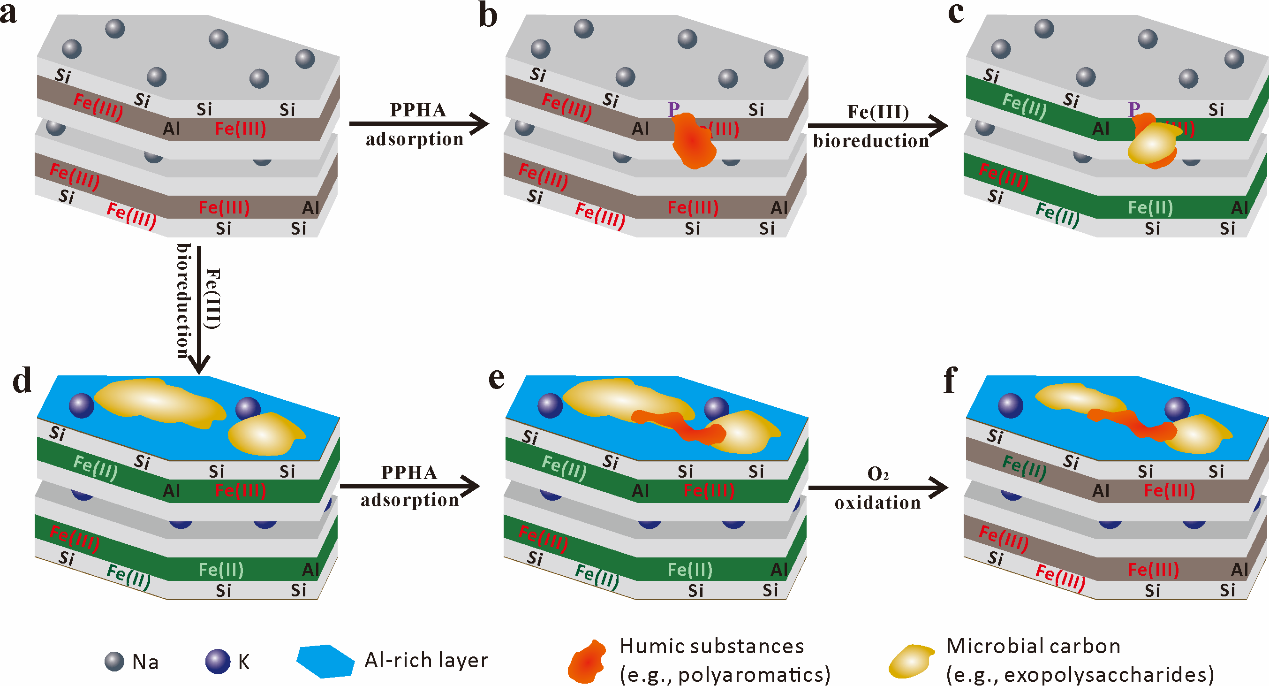
Figure 2. Summary schematic illustration of adsorption and reduction processes in NAu-2–HS–microbe experiments, as determined by ToF-SIMS spectra and PCA analyses. (a) In NAu-2, SiO occupies the basal surface, and some Na is adsorbed on the interlayer and basal surface, (b) only a small amount of PPHA is adsorbed on the edge of NAu-2, (c) during bioreduction, the adsorbed PPHA can maintain the integrity of the NAu-2 structure by acting as an electron shuttle, allowing Fe(III) reduction to proceed in a solid state, and resulting in the adsorption of some MC onto pre-adsorbed PPHA, (d) as a comparison, if NAu-2 is directly bioreduced without any PPHA pre-adsorption, NAu-2 may be partially dissolved, releasing some Al that can be redeposited to form an Al-rich layer on mineral surfaces, while MC can be adsorbed on such an Al-rich basal surface, (e) PPHA can be adsorbed to the basal surface of bioreduced NAu-2, and (f) further oxidation of Fe(II) does not significantly desorb (or consume) PPHA and MC, but MC is relatively less stable than PPHA in this process.
Title: Molecular determination of organic adsorption sites on smectite during Fe-redox processes using ToF-SIMS analysis.
Authors: Huang, Liuqin; Yu, Qun; Liu, Wen; Wang, Jungang ; Guo, Wenxiao; Jia, Endong ; Zeng, Qiang; Qin, Ruijun ; Zheng, Jianqiu ; Hofmockel, Kirsten; Dong, Hailiang ; Jiang, Hongchen*; Zhu, Zihua*.
DOI:https://doi.org/10.1021/acs.est.0c08407